The welding of duplex stainless steel S32750
Compared with Austenitic stainless steel, super duplex stainless steel has more Cr and Mo contents, which is beneficial to form ferrite and improve the corrosion resistance of the steel. The addition of Ni, N, Cu and Cu can improve the corrosion resistance of steel to non-oxidizing medium. Super duplex stainless steel has good weldability without welding hot and cold cracks. Under the influence of welding heat cycle, Ferrite increases and the grain size enlarge, while too slow cooling will also lead to the precipitation of harmful phase, which may destroy the balance between Austenite and Ferrite, affect the mechanical properties and corrosion resistance of welded joints. Here this article will introduce the welding process of S32750 stainless steel.
Welding methods
Tungsten argon arc welding is characterized by energy concentration, a small amount of heat input, easy to control the welding quality. Reasonable control of the welding heat input, multi-layer welding, multi-channel and low deposition rate, the tungsten electrode argon arc welding and auxiliary to 99.99% pure argon gas protection weld molten pool implementation super duplex stainless steel welding, can get better welding quality and good mechanical properties and corrosion resistance.
Welding materials
According to the chemical composition and mechanical properties of the base material, ER2594 wire is an ideal choice. The weld metal is allowed to be called “super duplex stainless steel” when the PRENE(pitting resistance equivalent value) is greater than 40.
Welding parameter
Sample operation is specified in ASME B31.1andASME Ⅸ。
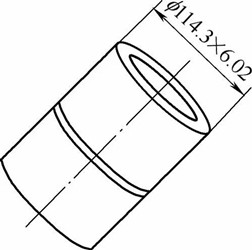
Firstly, take the base material sample S32750 pipe with the specification f114.3mm 6.02mm and open the V-shaped groove. The groove and weld bead is shown in the figure.

The welding material model is ER2594 with the specification f2.0mm. Note that too much current is easy to burn through, too little current is easy to cause incomplete fusion or incomplete welding. In the process of operation, the weld groove Angle can be appropriately increased to control the fusion ratio and adjust the metal composition of the weld.
Secondly, it is strictly prohibited to start the arc and test current on the surface of the base metal outside the groove to prevent arc damage to the base metal. The quality of the starting and ending arc should be guaranteed during welding. The same welding material and welding process as the root pass shall be used for welding positioning weld. The number of locating solder joints shall be 2, 3 or 4 points and shall be fixed on average. The thickness shall not exceed 2/3 of the pipe wall to ensure that the welding seam will not crack and remove defects during the formal welding process.
Welding shall be done in strict accordance with the relevant parameters selected. In order to make the welding can cover the actual construction of large-diameter, thick-wall pipe should be used as wide as possible range of parameters. Controlling interpass temperature less than 120 ℃, the welding heat input 1500 j/mm or less, on the premise of guarantee the quality of welding, as far as possible use small current, fast welding, small heat input and welding layer, bead for welding.
It is worth noting that the groove and the surface 50mm away from the groove shall be cleared before welding, and there shall be no water vapor, phosphating substances, carbon-containing materials (such as oil, paint, scale, rust, burr and halogen, etc.) and cracks, interlayer and other defects. Proper measures such as isolation and stacking should be taken to prevent the contamination of the super duplex stainless steel by iron elements. Super duplex stainless steel has good weldability and is not easy to produce hot cracks, been widely used in seawater and wastewater treatment equipment, papermaking, petrochemical equipment and other environments where require strict corrosion resistance.