The common heat treatment methods of copper alloy are homogeneous annealing, stress – free annealing, recrystallization annealing, solid solution and aging treatment. In order to prevent oxidation during processing, save the cost of pickling and obtain a bright surface, it is allowed to anneal copper alloy strip, wire and coil tube in a protective atmosphere or vacuum furnace, that is, bright annealing.
A large amount of O2, CO2 and H2O in the air will oxidize the surface of the copper alloy, which must be pickled before further processing. Heating in a protective atmosphere can reduce the oxygen content in the furnace and greatly improve the surface quality of the annealed copper alloy. Bright annealing process does not need pickling equipment, no environmental pollution, will not harm the health of personnel, reduce the metal loss and save costs, and greatly extend the service life of copper alloy strip, wire and coil.

Protective gas
Common protective gases are O₂, CO₂, CO, H₂, H₂ O and N₂. Among them, N₂ can be considered as an inert gas in the heat treatment temperature and does not participate in chemical reactions, while O₂, CO₂ and H₂ O are oxidizing gases, and CO and H₂ are reducing gases. the main components of surface oxidation of copper in the reaction are O₂ and H₂ O. Oxygen reacts with copper and zinc to form metal oxides. The equation is 4Cu + O₂ ==== 2Cu₂O.
Very small amounts of oxygen in the protective atmosphere are enough to oxidize copper and zinc. The oxygen content in the furnace must be less than 1ppm for the bright treatment of copper alloy, otherwise the alloy surface will oxidize. Because water vapor can oxidize copper alloys containing zinc, aluminum, lead, tin, beryllium and so on in heating, and the lower the temperature, the more obvious the oxidation. Therefore, the atmosphere in the furnace must be kept below -60 ℃.
The main protective atmosphere used in heat treatment of copper alloy is: high purity nitrogen, purifying exothermic atmosphere, nitrogen, ammonia decomposition gas; Pure hydrogen and so on. Among them, the high purity nitrogen itself has no reduction ability, the reducing atmosphere in the purification exothermic atmosphere and the nitrogen-based atmosphere has less CO and H2, and the reduction potential is low, so they are not suitable for the bright treatment of copper alloy. At present, the protective atmosphere is mainly ammonia decomposition gas and pure H2. 75% of ammonia decomposition gas is H2, and the remaining 25% is N2. This is because H2 has a good reduction and excellent heat transfer performance than nitrogen, high-speed temperature consistency and rapid cooling also increase the productivity correspondingly; With the change of the heat transfer effect, the temperature difference in the charge decreases, and the phenomenon of adhesion decreases. The density of hydrogen is very low, which can greatly reduce the energy consumption per unit and the resistance of hot air circulation, and reduce the noise of the furnace platform strong circulation motor, keeping it below 85dBA.
Lubricant
Lubricants also play an important role in achieving a good bright annealing effect. First, it must completely evaporate, without removing oxygen from the process of spotless heating, or the oxygen will react with the hydrogen in the protective gas to form steam, reducing the reduction potential. Secondly, mineral oil and emulsion are used as a lubricant in cold rolling of copper strip. The characteristics of the emulsion are good cooling effect, can obtain a large number of trace pressure, and make high-speed rolling possible, improve the productivity, but the emulsion has the defects of impurities and easy to be eroded, so the emulsion rolling strip must be annealed in a short time, otherwise, it will be corroded. At present, low viscosity mineral oil has been known as the main lubricant due to less impurities and volatile after heating, can achieve a good bright annealing effect.
Bright annealing equipment
The bright annealing processing equipment of copper alloy strip, wire and coil are mainly bell type annealing furnace. The process is not only to get a smooth surface and suitable mechanical properties, and without phenomenon of glue, which have a higher requirements for the performance and structure of annealing equipment.
- Good furnace temperature uniformity
Annealing equipment must have very good furnace temperature uniformity so that the annealing temperature of copper alloy is accurately controlled. Hooded annealing furnace has strong convection circulation system, effective recirculating fan, large air volume, high wind pressure, fast wind speed, excellent heat exchange effect, the furnace temperature uniformity is less than ±5 ℃, so that all the furnace charge can get uniform mechanical value and process value. At the same time, the annealing time is shortened and the productivity is improved.
The workload space is an all-metal enclosure. With the aid of a water-cooled rubber seal between the hearth flange and the inner cover flange, the circulating fan device achieves an absolute vacuum sealing space. There is no mechanical seal at the fan shaft and there is no possibility of leakage. Therefore, the dew point of the protective atmosphere can be maintained at -60 ℃ during the whole annealing process, which makes it possible for the bright annealed copper alloy.
First of all, the vacuum should be pumped and then the nitrogen should be sent to purge so that the atmosphere in the working space is as pure as possible, that is, it contains as low oxygen as possible. Test the tightness of the working space to find the leakage point so that there is no chance of mixing air and hydrogen. This vacuum process is essential for copper alloy wires and tubes. In the annealing process, the whole heating stage is cleaned by a protective atmosphere instead of a vacuum. Because the protective atmosphere can remove the evaporating lubricant more effectively than a vacuum to ensure the surface of the annealed workpiece is bright and clean.
- Unique combination cooling system
In general, each set of hood-type furnace is equipped with two hearth, a heating hood and a cooling hood. For optimum benefit, the furnace cooling time must be shorter than the heating time to allow sufficient time for vacuum displacement at the end of cooling, unloading, loading, and annealing in the next cycle.
Equipped with high efficient strong convection circulating fan, the heat transfer rate of convection is greatly increased, and the time of the furnace is greatly shortened during the annealing process of heating, heat preservation and cooling. In addition, the combined air/water cooling system, not only at the beginning of cooling, cooling hood blower suction air sprayed on the surface of the inner hood, has been cooling the inner hood to below 200 ℃, and then the water spraying device began to work, spraying water on the inner hood until the end of cooling. It not only prolongs the service life of the inner cover but also greatly shortens the cooling time.






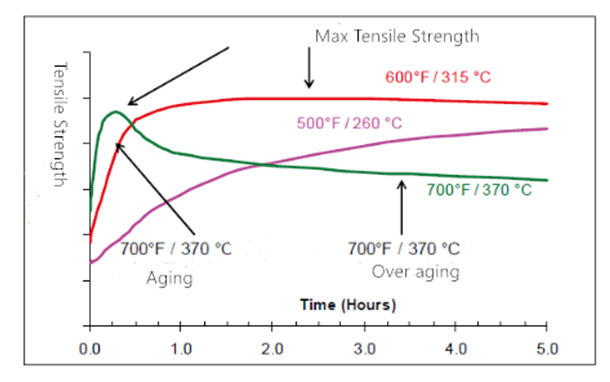

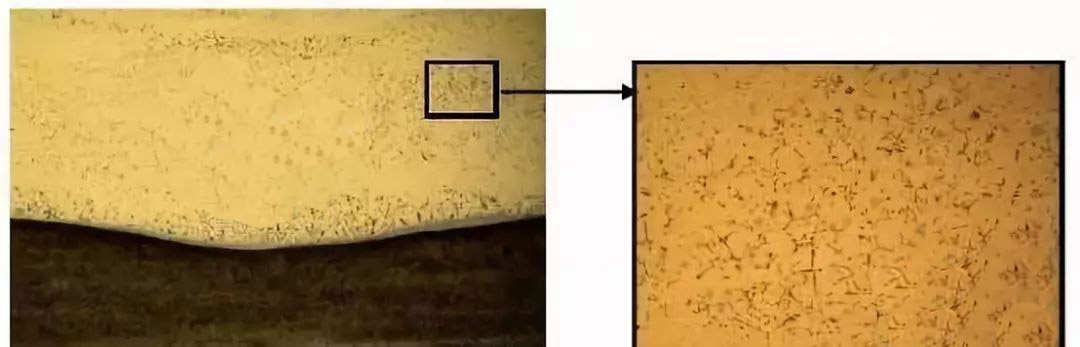 It is generally believed that the overcast in welds is caused by the high content of Fe in welds. When the liquid metal of infinite solution solidifies from high temperature to solid, the solubility of Fe decreases greatly, forming overcast in the weld, which will affect the performance of welding seams.
It is generally believed that the overcast in welds is caused by the high content of Fe in welds. When the liquid metal of infinite solution solidifies from high temperature to solid, the solubility of Fe decreases greatly, forming overcast in the weld, which will affect the performance of welding seams.









