Can copper and steel be welded together?
As we all know, copper and steel (iron) are two different metals. The thermal conductivity of copper is 7-11 times greater than that of ordinary carbon steel, and it is difficult to reach the melting temperature. When copper is melted, its surface tension is 1/3 less than that of iron, and its fluidity is 1-1.5 times greater than that of iron. Iron and copper are infinitely soluble in a liquid state and finite in solid-state and do not form intermetallic compounds. For the solid solution of iron and copper, the solubility of iron in copper at 650℃ is only 0.2%, and that of copper at 1094℃ is only 4%. In addition, the linear expansion coefficient of copper is about 40% larger than that of iron. The crystallization temperature range of iron-copper alloy is about 300-400℃, and it is also easy to form (Cu+Cu2O), (Fe+FeS), (Ni+Ni3S2) and another low-melting eutectic. The liquid copper or copper alloy has a strong permeability to the grain boundary of the steel near the crack zone. The characteristics of copper determine that the welding of steel and copper is often difficult.
- Welding heat crack.
- Intergranular penetration and penetration crack.
This generally occurs in the near-weld zone of the steel side matrix. The data show that the addition of Mn, Ti, V and other elements to the copper alloy or welding seam containing Ni, Al and Si can effectively reduce the tendency of penetration crack. For example, when the content of Ni is higher than 16% (mass fraction), no penetration crack will occur, while serious penetration will occur to the bronze containing tin. In addition, the microstructure of steel also affects, such as liquid copper can infiltrate austenite but not ferrite, so single-phase austenitic steel is prone to osmotic cracks, but not for Austenitic – Ferrite dual-phase steel.
- The weld overcast
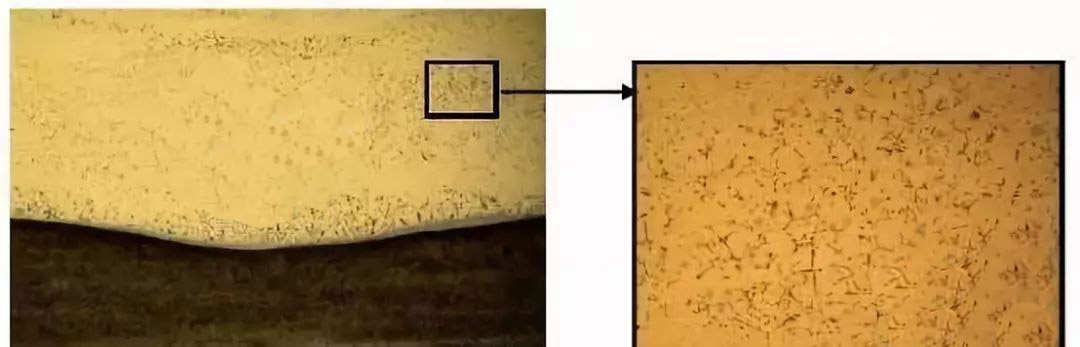 It is generally believed that the overcast in welds is caused by the high content of Fe in welds. When the liquid metal of infinite solution solidifies from high temperature to solid, the solubility of Fe decreases greatly, forming overcast in the weld, which will affect the performance of welding seams.
It is generally believed that the overcast in welds is caused by the high content of Fe in welds. When the liquid metal of infinite solution solidifies from high temperature to solid, the solubility of Fe decreases greatly, forming overcast in the weld, which will affect the performance of welding seams.
But because steel and copper have similar lattice types, lattice constants, and atomic radii at high temperatures, special welding techniques allow them to be welded together. It is generally believed that when Fe is 0.2%-1.1% in the weld, the weld structure is large α-phase, with poor crack resistance. With the increase of iron content, the weld was α+ε biphasic structure with the best crack resistance, especially when the Fe mass fraction was 10%-43%. Do you know how to weld stainless steel and copper?
Manual arc welding, argon arc welding and gas shielded welding can weld steel and copper and their alloys. It is recommended to use pure nickel or a nickel-based alloy containing copper to deposit the transition layer because of the strong crack resistance of nickel-based welds. Nickel element can greatly reduce or eliminate the copper and copper alloy permeable steel, which is helpful to eliminate the permeable crack in the heat-affected zone. In this experiment, pure copper 300mm×150mm×5mm C11700 copper plate and steel A 106 were taken as examples. After surfacing the transition layer, silicomanganese bronze wire 201 and wire 202 could be used as filler metal materials to strengthen the deoxidation of the melting pool.
Step 1. The oxidation film and oil stains on the surface of copper and steel metal base metal were cleaned up and polished, and then the copper side groove was processed to a side of 40° and the surface roughness Ra was 0.8m ~ 1.0m.
Step 2. The copper and steel metal base materials are heated in a box furnace. The heating temperature was 400℃ ~ 500℃ and kept for 30min ~ 45min.
Step 3. The copper plate and the carbon steel plate base material are filled with S201 red copper wire by tungsten argon arc welding (TIG) and fixed by spot welding. Then, the copper plate is connected by fusing and brazing, and the arc is shifted to the base material on the copper side (arc deviation is 10° ~ 25°). Parameters: current 140A ~ 160A, voltage 8V ~ 10V, protective gas He ~ Ar mixed gas, gas flow rate 15L/min; The volume ratio of He and Ar in the mixture of He ~ Ar is 8:2.
Step 4. Clean the welded joints with a wire brush until it has a metallic sheen and the welding is finished.
This welding method of copper and steel adopts He ~ Ar high energy protection gas to concentrate the line energy, which can shorten the residence time of high temperature in the melting pool and prevent the excessive melting of the substrate to make the copper and steel completely mix, spread and increase the copper content at the interface, resulting in the continuous infiltration of the steel side and the formation of low-melting eutectic heat cracks.
At the same time, He ~ Ar mixture of high-energy protective gas can also inhibit the combination of oxygen and copper, thus inhibiting the formation of oxide particles at the copper interface and preventing the formation of cracks. In addition, in the welding process, the arc is inclined to the copper side to ensure that the steel side is not melted, and the fusion and brazing joint are formed to avoid the excessive penetration of molten copper into the steel side and the formation of penetration crack, so as to reduce the high-temperature action time of the heat-affected zone and improve the plasticity and toughness of the welded joint.

















